send link to app
Balance The Chemical Equation app for iPhone and iPad
4.8 (
1088 ratings )
Education
Developer: Prachi Pimpalkhare
0.99 USD
Current version: 4.0, last update: 3 months agoFirst release : 10 Jan 2019
App size: 3.63 Mb
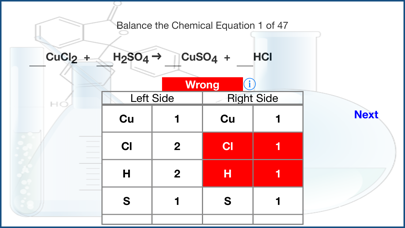
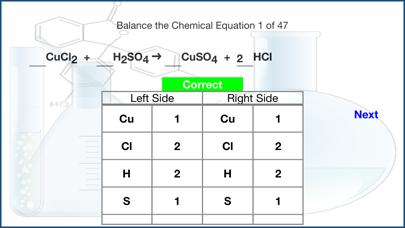
In chemical reactions, atoms are never created or destroyed.
The same atoms that were present in the reactants are present in the products as well and are merely reorganized into different arrangements.
In a complete chemical equation, the two sides (Reactants and the Products) of the equation must be balanced.